The following is a press release from the administration submitted to SOURCE media
***
[broadstreet zone=”51611″]
BOSTON – Today, December 9, the Baker-Polito Administration announced allocation and distribution plans for the first round of COVID-19 vaccine shipments to Massachusetts set to begin around December 15.
The state’s first shipment of 59,475 doses of the Pfizer vaccine was ordered from the federal government this past Friday and will be delivered directly to 21 hospitals across 8 counties, as well as to the Department of Public Health Immunization lab.
Doses will then be redistributed for access to 74 hospitals across all 14 counties for front-line medical workers.
The next 40,000 doses of Pfizer vaccine will be allocated to the Federal Pharmacy Program to begin vaccinating staff and residents of skilled nursing facilities, rest homes and assisted living residences.
Vaccine is being prioritized for these groups to maximize life preservation and to support the health care system.

Based on information at this time, Massachusetts is expecting 300,000 first doses of the vaccine to be delivered by the end of December.
The first vaccines, manufactured by Moderna and Pfizer, will require two doses administered 3-4 weeks apart.C
While all delivery dates and quantities are subject to change due to ongoing federal approval and allocation, the Administration plans to receive and distribute over 2 million doses to priority population groups by the end of March.
In collaboration with the COVID-19 Vaccine Advisory Group, the Administration designated groups of medical workers, first responders and residents most at risk for serious illness to receive the vaccine before the general population.
The Vaccine Advisory Group is made up of leading medical, infectious disease and public health experts as well as representatives from communities of color and representatives of high-risk populations. Communities of color and at-risk populations are prioritized throughout the process to maximize life preservation and to prevent serious complications from COVID related illnesses.
[broadstreet zone=”59947″]
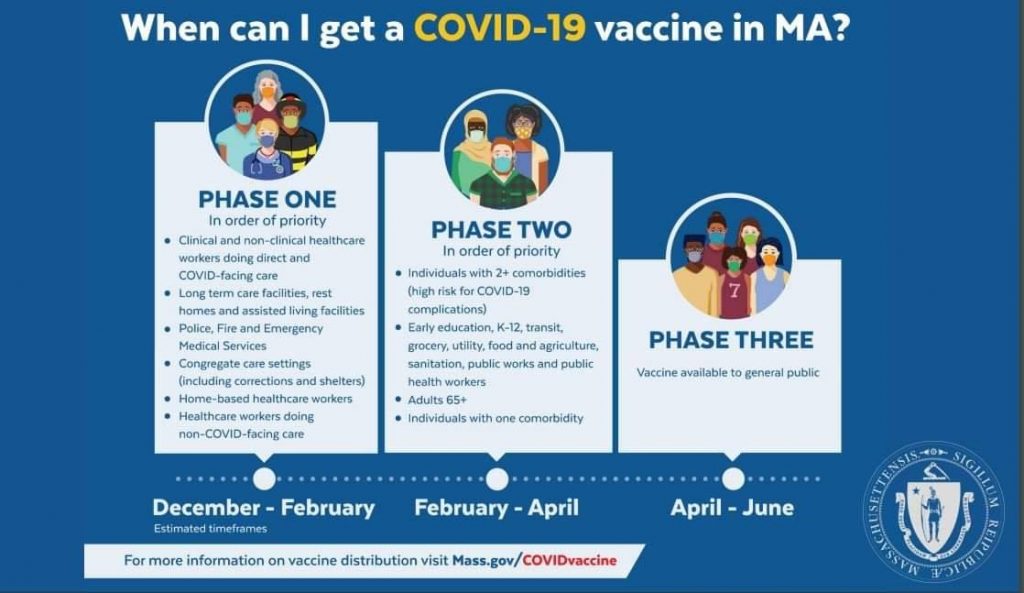
Anticipated Vaccination Phases and Timeline:
Phase One (December 2020-February 2021):In order of priorityClinical and non-clinical healthcare workers doing direct and COVID-facing careLong term care facilities, rest homes and assisted living facilitiesPolice, Fire and Emergency Medical ServicesCongregate care settings (including shelters and corrections)Home-based healthcare workersHealthcare workers doing non-COVID facing care
Phase Two (February 2021-April 2021):In order of priorityIndividuals with 2+ comorbidities (high risk for COVID-19 complications)Early education, K-12, transit, grocery, utility, food and agriculture, sanitation, public works and public health workersAdults 65+Individuals with one comorbidity
Phase Three (April 2021- ):Vaccine available to general public

The first shipments of the vaccine are expected to contain doses manufactured by Pfizer and Moderna.
While both Pfizer and Moderna vaccines are pending FDA emergency use authorization, Massachusetts will not distribute the COVID-19 vaccine until it receives this authorization.
Vaccines go through extensive testing, more than any pharmaceuticals, including extensive testing in clinical trials.
The FDA, which approves the vaccine, and the CDC’s Advisory Committee on Immunization Practices (ACIP), which will make its recommendation for use, must ensure any vaccine is both safe and effective for the public before approval and distribution.The infectious disease experts in the state’s academic medical centers have pledged to review the EUA data and provide an independent opinion about their safety and efficacy.
[broadstreet zone=”59948″]
All residents should visit www.mass.gov/COVIDVaccine to learn more or contact their medical provider for questions about their vaccination plans.
***